As the autumn mists descend and the first really heavy rain and wind storms of the winter are upon us, many gardeners start thinking about seeds and seed saving. Actually, it is already mostly too late for the 2020 seed season, but now is the time to look ahead and think about how you can position yourself to save seed next year.
Before doing this, it is worth taking a moment to consider why bother seed saving at all?
For a gardener or farmer, this might be about the pleasure and challenge of selective breeding for improved crop or yield. Choosing the seeds of your best specimens each year. Grow plants near to each other to encourage cross pollination and transmission of desirable genes, by saving and growing out of those seeds the next year. This is certainly a valid reason to want to save your own seeds each year.

Saving seeds from the best of your crops can be a financial boon and a pleasurable challenge to improve both crop and yield.
There is a political aspect to this as well. Hybrid seeds, those that give the biggest fruits or flowers or yield, produce seed which, while viable, does not breed true (may or may not resemble parent plant) and this forces farmers to purchase new seed every year for a consistent crop. This has huge financial benefit to the giant multinational seed corporations, but a huge financial impact on small farmers. Saving seed from open pollinated (non-hybrid) plants can be seen from this perspective as an act of defiance, autonomy and food sovereignty.
When it comes to a botanic garden, the meaning and significance of seed saving and seed banking is a little different.
Amongst other things, a botanic garden is committed to
- developing, documenting, verifying, maintaining, sharing, propagating and disseminating their plant collections
- serving as reference centres for plant identification, cultivar registration, nomenclature and plant exploration
What makes a botanic garden and what do they do?
Botanical gardens around the world are registered through Botanic Gardens Conservation International (BGCI) (www.bgci.org), which is based at Royal Botanic Garden, Kew in London. This organization accredits 3,650 botanic gardens around the world, 15 in Canada, five in B.C. and just one in the Comox Valley – Innisfree Farm!
In order to be registered, a botanic garden must demonstrate:
- a reasonable degree of permanence
- an underlying scientific basis for the collections
- proper documentation of the collections, including wild origin
- monitoring of the plants in the collections
- adequate labeling of the plants
- open to the public
- communication of information to other gardens, institutions and the public
- exchange of seed or other materials with other botanic gardens, arboreta or research institutions
and where possible
- undertaking of scientific or technical research on plants in the collections
- maintenance of research programs in plant taxonomy in associated herbaria
Botanic Gardens Conservation International (BGCI) and its member gardens around the world are also committed to promoting and promulgating the 16 targets of the Global Strategy for Plant Conservation (GSPC). Even in a small garden like Innisfree, all of our planning and development is predicated upon working towards these targets. (see appendix).
Kew Gardens, the home of BGCI and the institution where I was fortunate in enough to train in Botanic Garden Management, is also home to the Millennium Seed Bank (MSBP), one of the two biggest seed banks in the world.
Actually, it is hosted at Wakehurst Place, an hour or so south of London, where there was room and opportunity to dig vaults into the ground for long term seed storage. This beautiful old stately home is the original family home of Nicholas Culpeper, he of herbal medicine historic fame. It is ironic and exceedingly appropriate that a seed bank should be built here; he would be very pleased about it I am sure.

Wakehurst Place was the home of renowned botanist, herbalist, physician and astrologer, Nicholas Culpeper (1616-1654). Photo by Chanchal Cabrera
Local education and resources
I studied at Kew in 2013 and 2014, and we made field trips to visit Wakehurst Place and the Seed Bank. One of the most inspiring aspects for me is that when Kew identifies a species at risk or a habitat about to be lost and they go in to collect seeds, part of their mandate is to rehabilitate the land if possible and protect and conserve the in-situ populations. They also provide education and resources for capacity building in the country where they are working, to help the local people be able to develop their own Seed Bank of threatened indigenous plants.
When a seed collector returns to Kew, only some of the seeds are sent to the seed bank and the remainder are germinated and grown out. This allows for research into best germination and best growing practices, and with the plants that survive and thrive, Kew then repatriates them to the country of origin to grow out their own indigenous plants. This attention to indigenous knowledge and ownership, and the concerns around biopiracy is inspirational and reassuring.
Saving your seed
Below are some of the guidelines and recommendations for best practices that were shared with us at the Millennium Seed Bank and which can be applied to the cottage gardener as much as the farmer or botanist.
Assessing the population
For the most representative sample of a plant population, a seed collection should be sampled randomly and evenly across the extent of the population and ideally at least 50 many individual plants can be harvested.
- Do you know for sure that you have identified the species accurately?
- What is the extent of the population you want to harvest from?
- How many individual plants are there?
- Is the population damaged in any way?
- Is the population at reproductive stage? (IE: are there seeds available now?)
- Do other populations exist? Can you access them for further collection?
Equipment for seed collecting
Obviously not all of this is necessary if you are in your own garden or immediate neighbourhood, but it is still good practice to record everything you do. By keeping a garden log book or a harvesting/seed collecting logbook, successes and failures can be properly identified, tracked and learned from.
- GPS, compass, altimeter and maps –always record precisely where you are collecting
- Permits and licenses if required for picking or export; indigenous guide or local plant specialist
- First Aid kit
- Field identification guides/flora, camera (images of the material collected, the individual plant and the plant population at landscape level)
- Hand lens (x10 or x20 magnification)
- Secateurs, pruners, pocket knife with scissors, leather gloves
- Collecting buckets/tarpaulin
- Permeable cloth/paper bags – don’t put seeds in plastic bags or closed containers, as they can mold
- Herbarium press, card and blotter papers – best practice is to press a sample of the plant for confirmation of botanical ID later
- Field data recording sheets
Getting and drying your seeds
Collect seeds from the plants as late as possible, but before too many of them fall off and get lost. You will need to dry the seeds for a few weeks before you put them away for the winter. Separate the seed heads from the stems and leaves as much as possible. Some seeds will be inside pods that need to be broken open.
If they are in a fleshy fruit or seed pod, make sure to remove this so that you have dry seed. Spread seeds out in a single layer on a clean cotton cloth in a shallow tray and leave in a warm dry place. If they are quite moist, you may need to change this cloth when it is damp.
For seed heads that are already relatively dry, simply tie whole stems together in loose bundles and put the bundle with seed heads down, inside a paper bag or a cotton pillow case. Tie the bag shut securely with a rubber band and hang to dry. This way you don’t lose any seeds while the plant is drying. Be sure to label the bag right away.
Assessing your seed stock
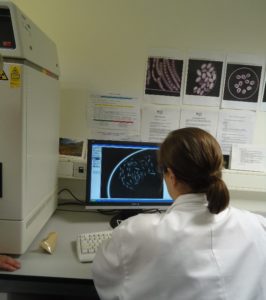
This shows a technician X raying a sample of the seed that has been collected to ascertain the viability. Some seeds may be sterile and this can be seen on the X Ray and if the percentage is high then further collections may be required. Photo by Chanchal Cabrera
Cut-test to assess seed quality (testing for empty seed)
- Cut 10-20 seeds, collected from several well-spaced individuals in the population.
- Use secateurs, scissors, nail clippers or similar sharp blade, cut along both axes if possible.
- Record the number of empty, infested and aborted seeds.
- Tiny seeds can be held on adhesive tape during sectioning.
- A hand lens of x10 or x20 magnification will help.
- Seeds that are known to be non-toxic can be crushed between fingernails or teeth if sectioning is difficult.
Cleaning seeds
In commercial seed production, growing sunflower or sesame seeds for example, there is significant mechanization of the seed cleaning process. However, for botanical collecting and for herbalists and gardeners, this is not likely to be necessary.
There are three main techniques for cleaning a seed collection.
- Using sieves of different mesh size to separate seeds from bulk material and smaller debris.
- Using a seed aspirator to remove similarly sized, but lighter/heavier, empty/infested seeds or debris.
- Hand-sorting, involving piece by piece removal of debris and rubbish.
For seeds are mixed with plant debris, pick out what you can. If debris is lighter than the seeds, blow gently to remove the debris. Toss a shallow container of seeds in front of a fan on a gentle setting to blow off more (winnowing). If winnowing does not remove chaff, pass the materials through one or more sieves with smaller and smaller spaces in the screens. You can use kitchen colanders and strainers as well as window screen materials, fabric, whatever is handy and has a suitable mesh size.
Drying seeds
The rate at which seeds age during the post-harvest period depends on the ambient relative humidity (RH) and the temperature. RH has a greater impact on seed longevity than temperature. For example, seeds will die faster under ambient conditions of 24°C and 70% RH than in a warmer, but drier, environment of 50% RH and 30°C.
As a general rule, an ambient daytime RH of 50% or less will help to maintain seed quality. Remember that RH rises as the temperature falls; a 10°C overnight fall in temperature could cause dry collections to absorb enough moisture to place them at high risk of ageing.
What is Relative Humidity (RH)?
Water is held in the air as water vapour. The amount of water vapour in the air at a given temperature is expressed as relative humidity.
% RH = water in a given mass of air x 100
– max.amount of water that could be held
What is moisture content (mc)?
Seeds are weighed before and after drying. Moisture content is expressed as weight of water removed, divided by either the fresh weight or the dry weight of seeds x 100. The main disadvantage of this method is its destructive nature – you know how much water your seed holds but the seed is now cooked and dead.
What is equilibrium Relative Humidity (eRH)?
Seeds will gain or lose moisture depending on the RH of the surrounding air, eventually reaching equilibrium, eRH is a measurement of the RH of the air at this equilibrium point.
A “safe” moisture level for collections in the field is not more than 50% equilibrium relative humidity (eRH). You should always take measures to promote drying of damp collections and ensure that moisture levels of drier collections do not rise. Seed life span approximately doubles for every 10% reduction in seed eRH.

This is one of the laboratories at the Millennium Seed Bank, with glass walls, so that the public can see in and watch the scientists at work. Photo by Chanchal Cabrera
“Dry” (<50% eRH), fully mature seeds
Seeds within dry fruits, collected in dry climatic conditions, may have already dried down to a safe level.
If ambient conditions are favourable:
- Loosely pack dry seeds in labelled bags and keep in a cool, well ventilated place.
- Spread bags out so that air circulates freely and place them in the shade – under the vehicle may be a good place – until all other fieldwork is complete.
- Dry seeds may need to be packed away at night to minimize moisture absorption.
- Similarly, if weather conditions change (e.g. rainfall) and ambient RH rises, protect collections from absorbing
“Wet” (50-100% eRH), fully mature seeds
Seeds which have only recently reached dispersal state or are in fleshy fruits, or seeds collected during the rainy season will be within this moisture range.
Wet seeds need to be dried as soon as possible. If ambient conditions are suitable (RH < 50%):
- Spread seeds out in a thin layer on newspaper in partial shade.
- Raise seeds off the ground if possible to allow air circulation.
- Re-pack seeds at night and place them within the vehicle to minimize moisture absorption as ambient RH rises.
Drying with desiccants
Any hygroscopic substance (silica gel, charcoal, dried seeds such as rice or maize, etc.) can be used to remove moisture from seeds, providing that the eRH of the desiccant is lower than the eRH of the seeds. If seeds are very wet, dry them for two to three days under ambient conditions before using the desiccant. This can be done even if the daytime ambient RH is relatively high (70- 80%).
Once the bulk water has been removed, transfer the seeds to a sealed container with the desiccant, allowing seed moisture to be reduced to a safe level (~30% eRH).
Use a silica gel:seed weight ratio of 1:1
To dry seeds to the same level using charcoal, use a weight ratio of 1:3.
Change the drying agent regularly to speed up drying time. Regenerate desiccants in the sun during the day and then use them to maintain seed drying at night, in sealed containers.
Storing the seed
Seed longevity increases as temperature is lowered (storage life approximately doubles for each 5ºC reduction in storage temperature). However, the increases in storage life become smaller as temperature is lowered.
Taking into account seed longevity, cost, safety and other factors (such as bio-physical stability), the Millennium Seed Bank Project (MSBP) recommends a seed storage temperature of about -20º C.

Here you can see the door to the vaults of the Millennium Seed Bank with its danger warning and the thermometer reading -20C degrees. Before entering, we were given protective insulated gear and we were only allowed to stay a few minutes. There were airlocks with positive pressure to prevent entry of pathogens and intense security screening as well. Photo by Chanchal Cabrera
Comparing seed cooling facilities:
- Domestic refrigerator – cool, cheap and replaceable, but it has a relatively high temperature for seed storage and small capacity
- Domestic deep freezer – cold, relatively cheap and replaceable (uprights are recommended, because they are drier), not recommended if more than 10m³ freezer volume is required
- Purpose-built cold room – cold, may use less energy than individual freezers, but there’s a high initial cost and maintenance is required
- Liquid nitrogen storage – deep cold, greatest seed longevity likely, suitable for very short-lived orthodox seeds, but it has small seed volumes and requires a ready supply of liquid nitrogen and an effective ventilation system
Because seeds are not forever, regular germination testing must be done and inventory management is obviously critical. If the seed becomes unviable, then all the rest of the work becomes irrelevant. In this way, a botanic garden where the plants can be grown in ex-situ situations becomes the very best seed bank possible; a living seed bank has resilience that no man-made manipulated environment can possibly give.
Appendix
Convention on Biological Diversity (CBD) and the Global Strategy for Plant Conservation (GSPC)

Part of the maintenance routine is to germinate seeds to ensure stock is viable. Photo by Chanchal Cabrera
The Convention on Biological Diversity (CBD) was initiated at the Earth Summit in Rio de Janeiro in 1992 and implemented late in 1993. It is an international treaty for the conservation of biodiversity, the sustainable use of the nature and the equitable sharing of the benefits derived from the use of genetic resources – 196 countries have signed it.
“The Convention seeks to address all threats to biodiversity and ecosystem services, including threats from climate change, through scientific assessments, the development of tools, incentives and processes, the transfer of technologies and good practices and the full and active involvement of relevant stakeholders including indigenous and local communities, youth, NGOs, women and the business community.”
To achieve these ambitious goals, the CBD developed 16 targets collectively called the Global Strategy for Plant Conservation. Begun in 2002 and renewed twice since then, a new report from September 2020 indicates that some countries have made considerable progress towards achieving many of them.
These include creating a World Flora Online combining expertise from 40 key institutions to create an open-access web-based compendium of the world’s 350,000 species of vascular plants and mosses (target 1) and a Global Tree Assessment (target 2). With currently one in five tree species globally known to be threatened with extinction, the Red List (risk threat) assessments for all the world’s tree species is of critical importance in helping prioritize national actions.

This image shows the vaults underground where seeds are stored on moving shelves that can be closed up tight to save space. Our time in here was limited due to the intense cold, but it was an extraordinary feeling to be surrounded by the world’s botanical gene pool. Quite moving and quite surreal. Photo by Chanchal Cabrera
The most progress internationally has been made towards Target 14 (public awareness of plant diversity), with targets 7 (in-situ conservation), 10 (invasive species) and 12 (sustainable use) being those where least progress has been made.
Click reference for updated GSPC
It is certainly encouraging that public awareness of plant diversity is increasing, but this is outpaced by the rate at which we are cutting down the forests and losing species around the world. With the in-situ conservation and sustainable use targets being the least achievable to date, this is a strong indicator of the importance and value of ex-situ cultivation and of saving seed.
16 targets of the Global Strategy for Plant Conservation (GSPC)
-
- Target 1: An online flora of all known plants.
- Target 2: An assessment of the conservation status of all known plant species, as far as possible, to guide conservation action.
- Target 3: Information, research and associated outputs, and methods necessary to implement the strategy developed and shared.
- Target 4: At least 15% of each ecological region or vegetation type secured through effective management and / or restoration.
- Target 5: At least 75% of the most important areas for plant diversity of each ecological region protected with effective management in place for conserving plants and their genetic diversity.
- Target 6: At least 75% of production lands in each sector managed sustainably, consistent with the conservation of plant diversity.
- Target 7: At least 75% of the world’s threatened species conserved in situ.
- Target 8: At least 75% of threatened plant species in ex situ collections, preferably in the country of origin, and at least 20% available for recovery and restoration programs.
- Target 9: 70% of the genetic diversity of crops, including their wild relatives and other socio-economically valuable plant species conserved, while respecting, preserving and maintaining associated indigenous and local knowledge
- Target 10: Effective management plans in place to prevent new biological invasions and to manage important areas for plant diversity that are invaded.
- Target 11: No species of wild flora endangered by international trade.
- Target 12: All wild-harvested plant-based products sourced sustainably.
- Target 13: Indigenous and local knowledge, innovations and practices associated with plant resources, maintained or increased, as appropriate, to support customary use, sustainable livelihoods, local food security and health care.
- Target 14: The importance of plant diversity and the need for its conservation incorporated into communication, educational and public–awareness programs.
- Target 15: The number of trained people working with appropriate facilities sufficient according to national needs, to achieve the targets of this Strategy.
- Target 16: Institutions, networks and partnerships for plant conservation established or strengthened at national, regional and international levels to achieve the targets of this strategy.

